

Our data showed that the secretion of neutrophils, regardless of their adhesion state, can contribute to this shift in the amino acid content.Ībbreviations: BCAAs: branched-chain amino acids Е2: 17 β-estradiol LPS: lipopolysaccharide from Salmonella enterica serovar Typhimurium fMLP: N-formylmethionyl-leucyl-phenylalanine.

Plasma of patients with diabetes is characterized by an increased content of branched-chain and aromatic amino acids and a reduced ratio of arginine/ornithine compared to healthy human plasma. In the presence of agents that impair cell spreading or adhesion (cytochalasin D, fMLP, nonadhesive substrate), neutrophils release the same amino acids, except for a sharp decrease in hydroxylysine and an increase in phenylalanine, indicating their special connection with cell adhesion.
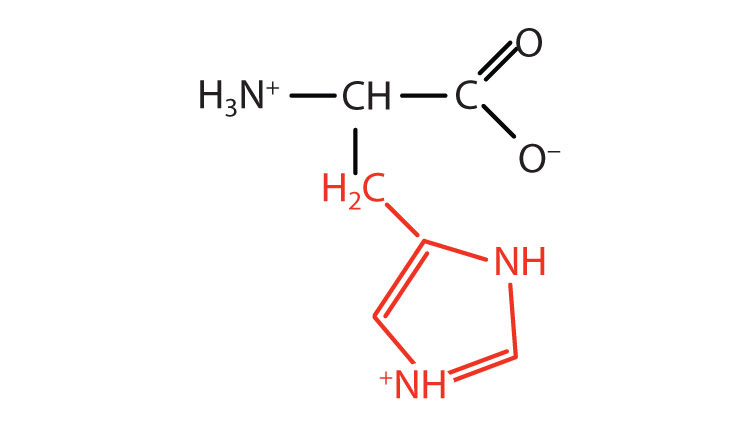
#POSITIVELY CHARGED AMINO ACIDS FREE#
Copyright 2002 American Chemical Society.Neutrophils release branched-chain (valine, isoleucine, leucine), aromatic (tyrosine, phenylalanine) and positively charged free amino acids (arginine, ornithine, lysine, hydroxylysine, histidine) when adhere and spread onto fibronectin. Item Type:įigures and tables reproduced here with permission from Biochemistry 41 (8), 2571-2579. Thus, we have discovered a means of specifically inactivating the interactions between the sMMO complex while preserving the catalytic activity of the hydroxylase active site which provides a new method of studying intercomponent interactions within sMMO.
Modification of positively charged groups on protein B had no effect on its function, consistent with the hypothesis that positively charged groups on the hydroxylase interact with negative charges on protein B. These results indicate that protein B and the reductase of the sMMO complex interact via positively charged groups on the surface of the hydroxylase to induce a conformational change that is necessary for delivery of electrons into the active site of the hydroxylase. It was shown that covalent modification of positively charged groups on the hydroxylase, which occurred at multiple sites, interfered with its physical and functional interactions with protein B and with the passage of electrons from the reductase. Covalent modification of arginine side-chains on the hydroxylase had a similar effect but, most remarkably, neither form of modification affected the activity of the hydroxylase via the peroxide shunt reaction. The cross-linker inhibited hydroxylase activity in the whole complex but this effect was due to covalent modification of primary amine groups rather than cross-linking. We investigated the effect of amine cross-linking on hydroxylase activity in order to probe the role of a gross conformational change that occurs in the hydroxylase upon binding of the other protein components. Also, in the presence of hydrogen peroxide, the hydroxylase alone catalyses substrate oxygenation via the peroxide shunt reaction. Whole-complex sMMO oxygenase activity requires all three sMMO components: the hydroxylase, the reductase and protein B. The soluble methane monooxygenase (sMMO) complex from Methylococcus capsulatus (Bath) catalyses oxygen- and NAD(P)H-dependent oxygenation of methane, propene and other substrates.


 0 kommentar(er)
0 kommentar(er)
